What Term May Be Used to Describe a Hydrophobic Substance
What is used to describe the amount of a harmful substance on fresh water. Or it may be used to describe a type of pharmaceutical preparation that is a medicated powder intended for.
What Are Hydrophilic And Hydrophobic Molecules Quora
6 pts Explain how the equilibrium constant can be used to describe the extent of an equilibrium reaction.
. What term may be used to describe a hydrophobic substance. A term that may be used to describe a substance that is hydrophobic is non polar. Hydrophobic refers to a fear of mixing or interacting with water under a certain set of reaction conditions.
The name hydrophobicity comes from two Greek words. Those that naturally repel water causing droplets to form are known as hydrophobic. This means lacking affinity for water tending not to combine with water or.
What is the term used to. Identify the components of a triglyceride below. Protein folding that places hydrophobic amino acids in the interior of the protein.
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. Main Difference Hydrophobic vs Hydrophilic Molecules. The lipid membrane of cells and organelles.
A replacing a straight chain alkyl group with a branched alkyl group may increase activity by filling up a hydrophobic pocket and increasing van der Waals interactions B increasing the chain length or size of an alkyl group may increase target selectivity if one target binding site is more spacious than another. But all compounds in nature do not mix with water. In other words hydrophobicity is a property of a substance that repels water.
A hydrophobic material has hydrophobicity and is so called hydrophobic. Examples of hydrophobic substances include fats oils waxes alkanes and other greasy substances. The substances that cannot mix with water are known hydrophobic substances.
A term that may be used to describe a substance that is hydrophobic is non polar. The term Powder may be used to describe. Non polar things are repelled by water.
Non polar things are repelled by water. It may be helpful to use examples Question. What term may be used to describe a substance that is hydrophobic.
ALipophilic BLipophobic CGlycerophilic DGlycerophobic. Describe how the hydrophobic effect achieves this result. Hydro which means water and phobos which means fear.
The physical form of a material that is a dry substance composed of finely divided particles. Water is a well-known solvent for the dissolution of most of the compounds we know. O This term is used to describe substances that are insoluble in water o Molecules are hydrophobic if they do not have negative or positive charges and are nonpolar o All lipids are hydrophobic including fats and oils o Hydrophobic molecules dissolve in other solvents such as propanone acetone.
A hydrophilic molecule or substance is attracted to water. A term that may be used to describe a substance that is hydrophobic is non polar. Which of the following may be used to determine protein utilization.
Non polar things are repelled by water. Water is a polar molecule that acts as a solvent dissolving other polar and hydrophilic substancesIn biology many substances are hydrophilic which allows them to be dispersed throughout a cell or organismAll cells use water as a solvent that creates the solution known. Fatty acids contain both a ____ group and a ____ group.
A term that may be used to describe a substance that is hydrophobic is non polar. The substances that can mix with water are called hydrophilic substances. Oil sheens seen on the ocean following an oil spill.
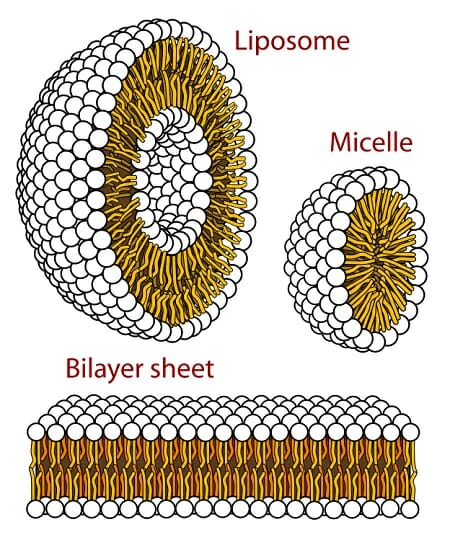
Examples include fatty acids fats phospholipids steroids and prostaglandins. A hydrophobic organic compound composed mainly of carbon and a high ratio of hydrogen to oxygen is a ____. Instead of being exposed to water hydrophobic molecules are nonpolar molecules that combine together to form micelles.
The separation of salad dressing. Which statement characterizes the lipase enzymes. 6 pts The hydrophobic effect is the process by which nonpolar substances are excluded from an aqueous solution.
Salivary gland lipase lingual lipase plays an active role in fat digestion in infants. Micelles are small spheres formed by the joint action of surfactants or detergents and water. Non polar things are repelled by water.
Internal ie oral powder external ie topical powder use. Which substance is involved in the clotting of blood. The term hydrophobic comes from the Greek and is translated as having a horror of water or water fearing.
In chemistry it refers to a substances ability to resist water. Materials with a special affinity for water those it spreads across maximizing contact are known as hydrophilic. Hydrophobicity is a term used in general science to describe a substances capacity to resist water.
The word hydrophobic literally means water-fearing and it describes the segregation of water and nonpolar substances which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between. All of these are correct.

Hydrophobic Definition Examples Molecules Substances
No comments for "What Term May Be Used to Describe a Hydrophobic Substance"
Post a Comment